How do Australia’s streamlined regulatory process and global tax incentive apply to your toxicology studies?
There are many benefits to conducting drug development studies in Australia, especially to timelines and expenses, without having to sacrifice quality. These unique incentives apply to all Research and Development, manufacturing, non-clinical and clinical studies required for the drug development process.
Benefits of Conducting Studies in Australia: Speed
One of the primary benefits to conducting drug development studies in Australia is speed. As long as your nonclinical program is robust, you can receive approval to initiate Phase 1 clinical trials in Australia within 6-8 weeks. Furthermore, you can conduct your Phase 1 clinical trials prior to your IND submission. This provides a significant advantage over development timelines in other locations as well as to the strength of your IND submission. Learn more about the process.
However, having a robust nonclinical program is critical for achieving this timeline. Even though the “Australian Advantage” is fast, efficient, and streamlined, it does not mean that safety studies can be skipped. You still need to conduct a very robust toxicological package under good laboratory practice (GLP) conditions, as the industry has to conform to the same high quality standards regardless of location. Agilex Biolabs operates under OECD GLP conditions, which is an equivalent standard to FDA GLP but is acceptable for submission to Australian, US and European regulatory authorities.
GLP nonclinical development generally includes pivotal safety studies, or in other words, studies that determine whether a drug will be safe in humans. That includes safety pharmacology and toxicology, including pharmacokinetics, toxicokinetics, and potentially bridging safety studies if there is precedent. Here is a depiction of a typical drug development program:
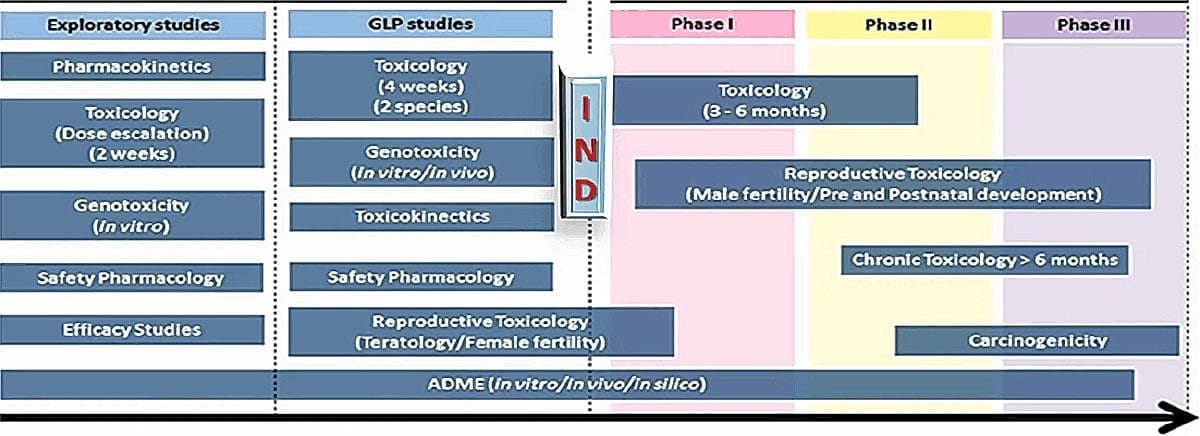
The drug may not be completely purified at this stage, but that’s acceptable because the toxicology studies are conducted with impurities present. That actually provides much broader safety coverage so the studies don’t need to be repeated once it’s been purified. On the other hand, if a drug is reformulated and produces a new chemical entity, it would potentially require toxicology or bridging studies to be repeated.
Efficiency Benefits Specific to Toxicology Studies
We discussed the streamlined approval process for initiating human trials, but what about efficiency benefits for animal studies? Agilex has access to a local animal ethics committee for approval of our animal studies, which operates on a rolling monthly approval schedule. Application approval is received within 2-3 weeks and the turnaround time for approval of animal use is about 6 weeks. Animals are available on a weekly basis from our supplier, so we can initiate the study within 6-8 weeks from signing the contract, subject to availability of test article.
What if we’re working with something other than a standard small molecule? Are there streamlined approval processes for highly regulated drugs? What about GMOs?
Yes, Australia also has a streamlined approval process for scheduled drugs and genetically modified organisms (GMOs). Agilex has a local license to hold highly regulated drugs, which include US Schedule 1 drugs (known as Schedule 9 drugs in Australia) such as cannabinoids and psychedelic drugs with high potential for abuse. We can add regulated drugs to our license within 2-3 weeks versus 6 months for US DEA approval. Approval for the use of GMOs can be obtained through our local biosafety committee within 4-6 weeks of application submission.
Additional Benefits: 43.5% Cash Rebate for R&D Expenses
Another benefit to conducting drug development studies in Australia is the cost. Companies with a turnover of less than $20 million AUD can receive a cash refund for eligible expenses incurred within Australia, which includes toxicology. Furthermore, offshore study expenses can be eligible for a cash rebate if the service is not available in Australia.
For instance, certain in vivo animal models are simply not available for studies in Australia. At Agilex, we generally focus on rodent studies and collaborate with Attentive Sciences in the US for other species. This allows multiple test systems, such as mice, rats, guinea pigs, ferrets, hamsters, mini pigs, canines and larger test systems, to be available and may still be eligible for the cash rebate.
Despite the difference in time zones, we’ve streamlined our collaborations to allow seamless research between our sites. You can even work through a single point of contact for all of your studies. By establishing an agreement with Agilex and having your drug material ready for testing, both Agilex and Attentive can provide quick start timelines to enable you to stay on track with your internal regulatory path and timeline requirements.
A Human Research Ethics Application (HREA) can also be submitted in order to only have to obtain one regulatory approval for multiple sites. This process works under the National Mutual Acceptance (NMA) system.
Example: US Drug Developer Conducting Studies Through Agilex
Let’s take a look at a real world example of someone putting what we’ve outlined into actual practice. A US-based biotechnology company developing a small molecule intended for use in oncology required a full IND-enabling GLP tox package to support their FIH studies in Australia. They initially contacted Agilex Biolabs who then provided an introduction to Attentive Science. Agilex and Attentive co-developed a proposal, which was accepted by the client and contracted through Agilex.
This allowed the client to seamlessly run their rodent studies in Australia, dog studies and safety pharmacology in the US, and bioanalysis for both in Australia, all under one contract. There were weekly meetings with Agilex, Attentive and the client, enabling a truly collaborative approach to the program, which was both highly successful and easily executed despite the difference in time zones. This is just one of many examples occurring year-round at Agilex.
Selecting an Australian CRO to Conduct Your Studies
The cash rebate, along with additional cost savings stemming from more efficient timelines, allows developers to utilize high quality contract research organizations (CROs) at a lower cost than in other countries. Selecting service providers with expertise and a reputation for high quality data allow you to gain additional benefits to efficiency by utilizing the best data for your development strategy as well as regulatory submissions.
Agilex Biolabs has over 25 years of experience supporting and accelerating preclinical and clinical trials from around the world. We provide high quality toxicology services, including exploratory toxicology and pharmacokinetic studies up to GLP toxicology studies, to support your entry to first in human clinical trials. We have in-house bioanalytical services support for both small molecules using LC-MS/MS-based methods as well as immunoassay (PK) and immunogenicity (ADA) methods for large molecules, pharmacodynamic assays for cell and gene therapy or immunology programs plus antibody titer assays for vaccine immune response.
We also have an FDA inspection history dating back to 2011 and our biomedical lab has ISO 17025 accreditation with both OECD GLP and ISO standards monitored by the National Association of Testing Authorities, which is the Australian government’s GLP compliance monitoring authority.
In addition, we always welcome sponsor audits. Both international and local clients are welcome to visit in person or via remote audit. The pandemic has reduced the number of in-person visits lately, but we’re looking forward to showing customers around our world class facilities again, which were actually used to conduct four GLP rodent studies to support the clinical development of three novel COVID-19 vaccine candidates.
To learn more about conducting your drug development program in Australia or about Agilex Biolabs, contact our experts today.



