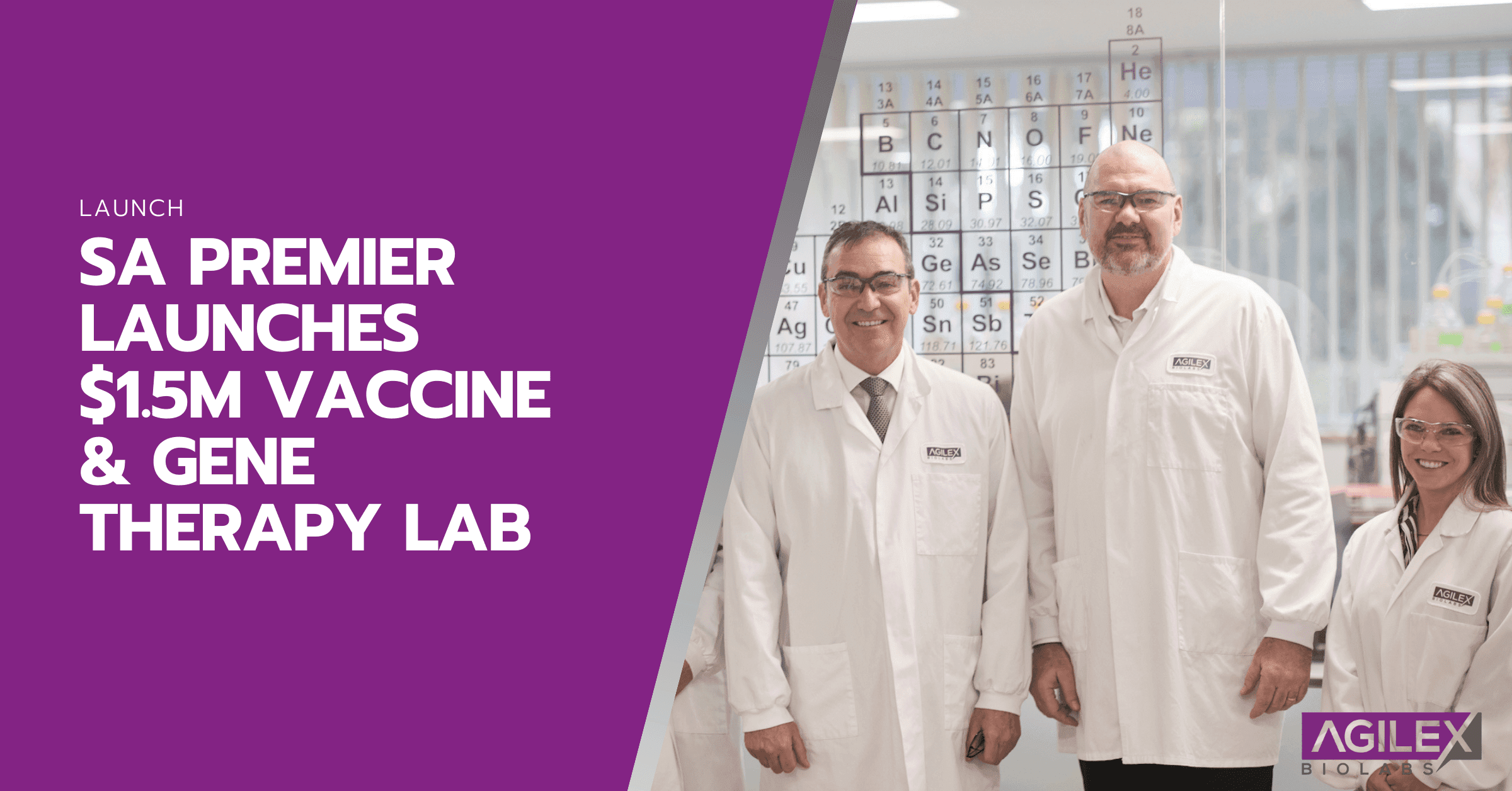
Agilex Biolabs, Australia’s largest and most technologically advanced regulated bioanalytical laboratory for clinical trials today announced that the Premier of South Australia the Hon Steven Marshall MP has launched its new $1.5m vaccine and immuno-biology laboratory. The facility, the most sophisticated in APAC, will attract biotechs and pharma from around the world for advanced clinical research.
Over the past 2 years, Agilex Biolabs has invested more than $3.5m in technology and systems at the APAC headquarters in Adelaide.
Over the past 2 years, Agilex Biolabs has invested more than $3.5m in technology and systems at the APAC headquarters in Adelaide.